How can we keep the corrosion away from Raymond mill surface? Raymond mill surface corrosion prevention is very crucial to mill life.
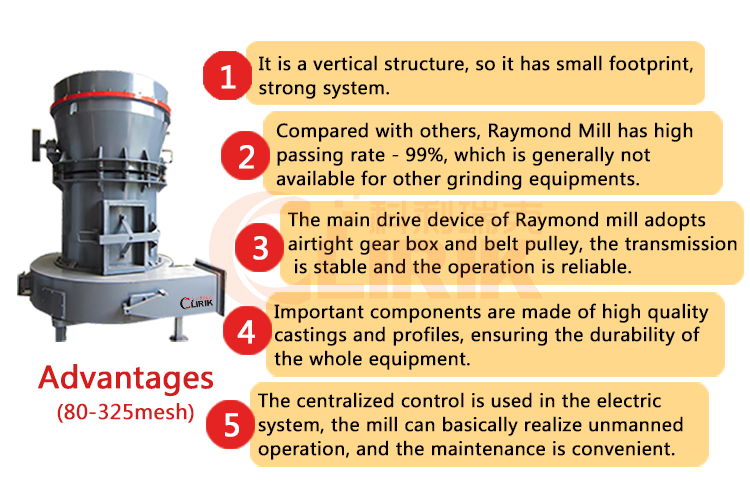
This kind of mill is a technical innovation based on the pendulum mill. The technical performance Raymond mill have been greatly improved in comparison with the pendulum mill. It is a new type of high-efficiency and energy-saving stone grinding machine. The fine grinder is suitable for grinding limestone, calcite, marble, talc, gypsum, barite, gypsum, fluorite, zeolite, manganese ore, ilmenite, phosphate rock, bentonite, starch, kaolin and other non-flammable and explosive materials with hardness below Mohr 7 and humidity below 6%. Fineness can be adjusted as required.
In order to improve the wear resistance and corrosion resistance of Raymond mill and play a decorative role, surface coating technology based on metal material coloring technology has been widely used.
Under natural conditions, a layer of oxide film is formed on the surface of iron and steel parts due to the contact with oxygen in the air. Depending on the physical properties, surface states and oxidation conditions of the metal itself, the oxide films formed on the metal surface will be different, some of them are thin, some are compact and complete, and some are loose and incomplete. The formation of a compact and complete oxide film with a certain mechanical strength on the surface of steel can be accomplished by chemical or electrochemical methods. There are many oxidation methods for iron and steel, such as alkaline oxidation, alkali-free oxidation, high temperature gas oxidation and electrolytic oxidation. At present, alkaline oxidation method is widely used. The surface of steel is oxidized to form a certain thickness of oxide film, showing a special oxidation color - blue-black, so it is generally referred to as "blue" or "black". The oxide film made of iron and steel has beautiful color, no hydrogen, elasticity and thin film, which has certain effect on eliminating the stress formed after heat treatment.
The mechanism of alkaline oxidation of Raymond mill is that steel parts are treated in solution containing sodium hydroxide, nitrate or nitrite at a certain temperature. When the solution temperature approaches the boiling point, the steel interacts with the oxidizing alkali concentration as follows: the steel begins to dissolve, forming sodium ferrite and sodium ferrite. At the same time, some sodium ferrite interact with each other to form ferrous oxide (brown red precipitates).The appearance and protective properties of the oxide film vary with the thickness of the oxide film. However, whether the film is thick or thin, the main component of the oxide film is magnetic iron oxide.